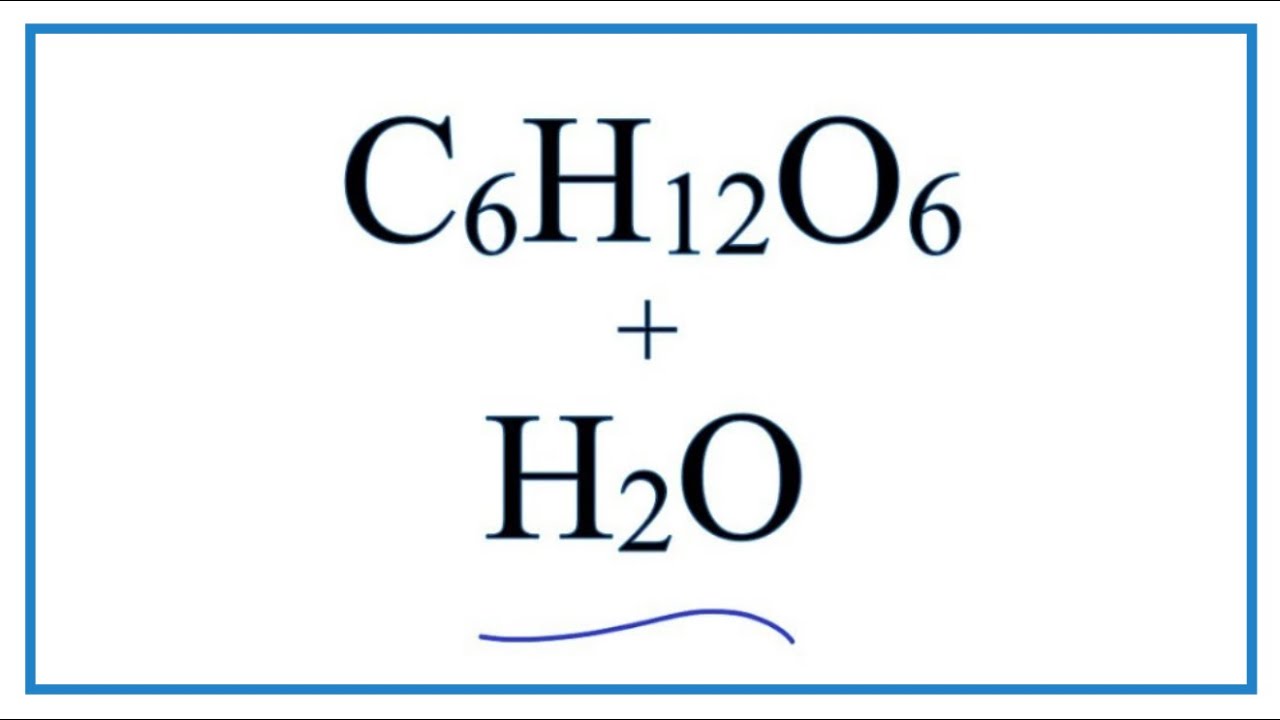
Cst review carbon crystals life molecules Solved: how many ions are produced when c6h12o6 dissolves in water? Molecules cst glucose
First Class Photosynthesis Equation Unbalanced Important Formulas Of
6co2 + 9h2o —– c6h12o6 +6o2 + 3h20 is this a balanced chemical equation
Is c6h12o6 (glucose) ionic or covalent/molecular?
First class photosynthesis equation unbalanced important formulas ofEnter title jeopardy template C6h12o6 lewis structure18 g of glucose c6h12o6 is dissolved in 1 kg of water in a saucepan. at.
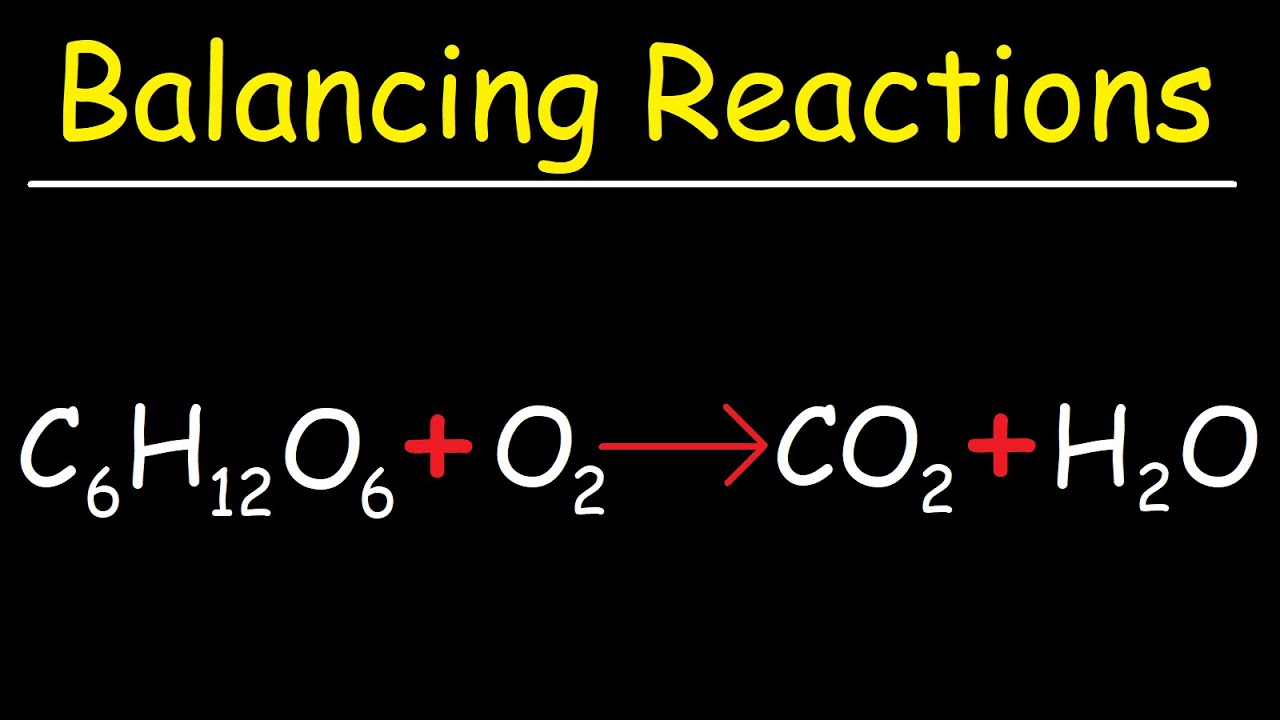
How to balance c6h12o6 + o2 = co2 + h2o (combustion of glucose plus18 g of glucose (c6h12o6) is dissolved in 1 kg of water in a saucepan Glucose (c6h12o6)Solved: what mass of glucose, c6h12o6, must be dissolved in 150.0 g of.

Dissolving substances in water at leo franklin blog
Glucose co2 h2o oxygen combustion plus o2 balanceC6h12o6 + 6o2 → 6co2 + 6h2o the reaction between c6h12o6 and o2 is Glucose structure molecular properties compounds monomer massHow many grams of glucose c6h12o6 should be dissolved in 0.5 kg of.
Glucose c6h12o6- chemical formula, structure, composition, properties, usesHow to balance c6h12o6 + o2 = co2 + h2o (combustion of glucose plus Balancing chemical equation co2+h2o=c6h12o6+o218g of glucose, c6h12o6, is dissolved in 1kg of water in a saucepan. at.

The molar mass of glucose (c6h12o6) is 180 g/mol.if 90 g of glucose is
Particle diagram of sugar dissolving in waterIs glucose polar or nonpolar (c6h12o6) Glucose molecule structure. the molecular formula for glucose isC6h12o6 lewis structure.
Chemical formula of glucose and fructose18 g of glucose, c6h12o6 (molar mass = 180g/mol ) is dissolved in 1 kg Co2 h2o o2 equation glucose dioxide balancingGlucose molecule structure.
Ions in aqueous solution infographic diagram showing, 44% off
Solved: draw a more accurate representation of one unit of c6h12o6Lewis dot structure of c6h12o6 C6h12o6 glucose molecule royalty free vector imageSolved: 5.67 grams of glucose (c6h12o6) is dissolved in 25.2 grams of.
18 g of glucose, c6h12o6 (molar mass = 180g/mol ) is dissolved in 1 kg .